Brief description of the results achieved so far by the University of Calabria (UNICAL) Unit
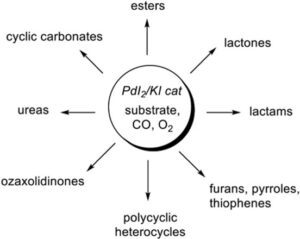
The UNICAL Unit has been studying novel processes for the catalytic incorporation of CO into simple substrates for the direct synthesis of high value added products. This research activity (involving in particular the use of a simple and efficient catalyst consisting of PdI2 in conjunction with KI under oxidative conditions, Scheme 1), is based on the long-lasting experience acquired in the course of many years in this field by this Unit and by the PI, as recently outlined in an invited review published in Tetrahedron Chem:
Scheme 1
The PdI2/KI-catalyzed oxidative carbonylation reaction, first published in 1992, has in fact demonstrated to be a powerful methodology for the multicomponent and sustainable synthesis of high value added carbonylated compounds (esters, amides, lactones, lactams, ureas, oxazolidinones, cyclic carbonates, ecc.) starting from simple starting materials (alkynes, alcohols, amines, etc) in combination with carbon monoxide as the simplest and atom-economical source of the carbonyl group. With this important methodology carbonylated derivatives can be produced in one step by the catalytically assembly of several simple units in ordered sequence. Moreover, the method employs the greenest and most convenient oxidation agent available (that is, oxygen from air) with formation of water as benign coproduct.
B. Gabriele, Palladium iodide catalyzed oxidative carbonylations. Tetrahedron Chem 2024, 12, 100107 (https://doi.org/10.1016/j.tchem.2024.100107).
Synthesis and Photochemical Characterization of Indolizine Fluorophores Obtained by a Multicomponent Palladium Iodide- Catalyzed Oxidative Aminocarbonylation Approach
The UNICAL Unit has developed a multicomponent approach to novel N,N-disubstituted 2-(indolizin-3-yl)acetamide derivatives with fluorescent properties starting from simple and readily available building blocks [2-(pyridin-2-yl)pent-4-yn-1-carbonyl compounds, CO, amines, and oxygen] through a sequential PdI2/KI-catalyzed oxidative aminocarbonylation-cyclization-aromatization process (Scheme 2). The indolizine core is present in many molecules that display a wide range of pharmacological properties. The optical properties of representative 2-(indolizin-3-yl)acetamide products have also been investigated.
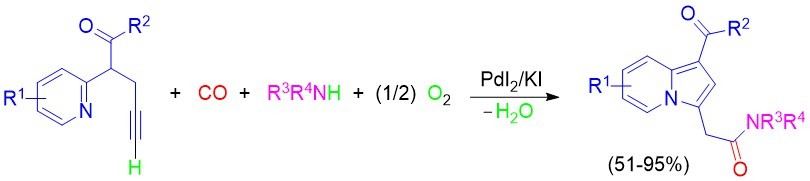
Scheme 2
I. Ziccarelli, R. Amuso, R. Mancuso, A. Maggiore, G. Lamberti, P. Vitale, V. Maiorano, L. Veltri and B. Gabriele, Synthesis and photochemical characterization of indolizine fluorophores obtained by a multicomponent palladium iodide – catalyzed oxidative aminocarbonylation approach. Eur. J. Org. Chem. 2024, 27, e202400013 (https://doi.org/10.1002/ejoc.202400013).
Synthesis of 2-oxazolidinones by oxidative carbonylation of b-amino alcohols under heterogeneous catalysis conditions
A novel PdI42—supported on a hybrid material based on imidazolium modified polyhedral oligomeric silsesquioxanes (POSS-Imi) grafted on amorphous silica (SiO2) has been prepared through a straightforward synthetic procedure allowing to accede to high local concentration spots of palladium sites surrounding the POSS core. The newly developed heterogeneous catalyst proved to be effective in the oxidative carbonylation of a variety of β-amino alcohols to give 2-oxazolidinone derivatives with antimicrobial activity in fair to high isolated yields (63-86%), as shown in Scheme 3. The recyclability of the catalysts has been successfully verified for four consecutive runs. ICP–OES analysis of some representative products evidenced a low metal contamination (palladium content <1 ppm), making the approach suitable for pharmacological applications, where a degree of palladium contamination < 10 ppm is required.
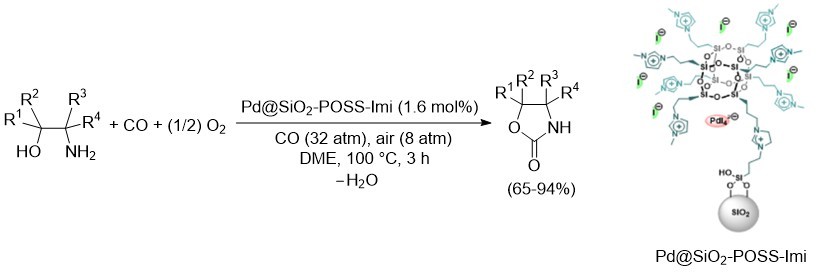
Scheme 3
I. Ziccarelli, R. Mancuso, M. Novello, A. De Salvo, C. Calabrese, L. Valentino, A. Pettignano, M. Gruttadauria, F. Giacalone and B. Gabriele. A Palladium tetraiodide supported catalyst for the oxidative carbonylation of b-amino alcohols to 2-oxazolidinones. ChemCatChem 2025, 17, e202401841 (https://doi.org/10.1002/cctc.202401841).
Synthesis of 2-(benzofuran-2-yl)acetamides by catalytic coupling of 1-(2-(allyloxy)phenyl)-2-yn-1-ols and isonitriles
A new catalytic approach to the synthesis of the 2-(benzofuran-2-yl)acetamides – an important sub-class of benzofuran derivatives with antifungal and anticonvulsant activities – has been developed. The new method is based on the use of Pd(PPh3)4 as simple and commercially available catalyst and readily available 1-(2-(allyloxy)phenyl)-2-yn-1-ols and commercially available isonitriles (compounds isoelectronic with carbon monoxide) as starting materials. 2-(Benzofuran-2 yl)acetamides have been obtained in fair to high yields (60–80 %) over 19 examples under mild reaction conditions (Scheme 4). The reaction mechanism has been corroborated by detailed DFT studies, and the structures of representative products have been confirmed by X-ray diffraction analysis.
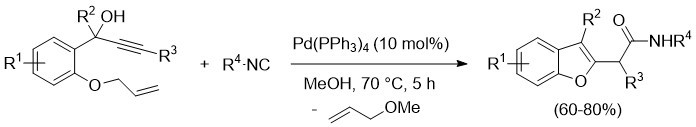
Scheme 4
R. Mancuso, P. Russo, M. Prejanò, A. Palumbo Piccionello, C. Cuocci, T. Marino and B. Gabriele, Catalytic coupling of 1-(2-(allyloxy)phenyl)-2-yn-1-ols and isonitriles for the synthesis of 2-(benzofuran-2-yl)acetamides: A combined experimental and theoretical study. J. Catal. 2024, 437, 115659 (https://doi.org/10.1016/j.jcat.2024.115659).
Brief description of the results achieved so far by the University of Parma (UNIPR) Unit in collaboration with the Unical Unit
The activities focused on the reduction of carbon dioxide (CO₂) to carbon monoxide (CO) and its application in organic synthesis, particularly in catalytic carbonylation processes. Given the environmental issues caused by increased CO₂ levels, finding sustainable carbon harvesting methods is crucial. Initial experiments involved selecting suitable transition-metal catalysts, particularly palladium-based complexes, for carbonylation reactions. A noteworthy protocol was developed by Stephen Buchwald, enabling the synthesis of amides from aryl bromides using a palladium acetate catalyst. The reaction was replicated but we found that using aryl iodides improved reaction rates. Further investigations were conducted on alkoxycarbonylation of alkynes, leading to high yields of methyl methacrylate (MMA) under modified conditions. The oxidative carbonylation of 2-alkynylanilines was also explored, demonstrating the potential for selective product formation.
The team then investigated CO₂ reduction pathways, specifically using tris(trimethylsilyl)silane as a reducing agent. Initial experiments showed promising results, with CO generation leading to the successful formation of aryl amides. However, the methoxycarbonylation of phenylacetylene yielded only trace amounts of the desired product, indicating slower reaction kinetics. In oxidative carbonylation experiments, product yields decreased compared to pure CO reactions, suggesting room for optimization.
The UNIPR Unit in collaboration with the UNICAL Unit has also studied and developed the non-oxidative carbonylation of propargylic ureas to give high value added 5-oxo-2,5-dihydro-1H-pyrrole-1-carboxamides with significant anti-HIV properties (Scheme 5). This reaction will be further studied for the possibility to employ CO2 with in situ reduction to CO, since there are no oxidants involved, this process will not interact with the reductive silane compound.
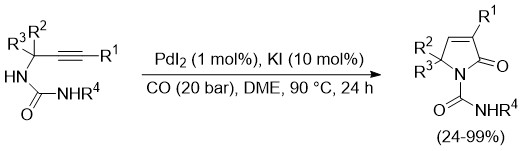
Scheme 5
D. Schiroli, A. Voronov, F. Pancrazzi, N. Iraci, S. V. Giofrè, B. Macchi, V. Stefanizzi, R. Mancuso, B. Gabriele, P. P. Mazzeo, L. Capaldo and N. Della Ca’, Direct Access to α,β-Unsaturated γ-Lactams via Palladium-Catalysed Carbonylation of Propargylic Ureas. Adv. Synth. Catal. 2025, 367, e202401183 (https://doi.org/10.1002/adsc.202401183).
The research conducted by the UNIPR UNIT also focused on the direct conversion of CO2 into value-added compounds without the intermediate step of producing CO. This resulted in an unprecedented one-pot, two-step synthesis of highly functionalized 1,3-dienes (Scheme 6).
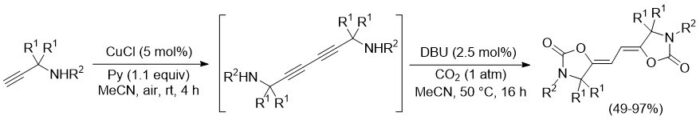
Scheme 6
F. Mele, A. M. Constantin, F. Sacchelli, D. Schiroli, P. P. Mazzeo, G. Maestri, E. Motti, R. Maggi, R. Mancuso, B. Gabriele, F. Pancrazzi and N. Della Ca’, Sequential Glaser reaction–diastereoselective cyclocarboxylation of propargylamines with CO2: A green catalytic access to bis-oxazolidinone-dienes and their topochemical polymerization”. Green Chem. 2024, 26, 6429-6435 (https://doi.org/10.1039/D4GC00818A).
In conclusion, the research successfully established an in situ method for CO₂ reduction to CO, which can be utilized in aminocarbonylation and oxidative carbonylation reactions. While some processes need further refinement, the findings indicate a feasible approach to combining CO₂ reduction with organic synthesis.
The IC-CNR research Unit has carried out the following activity
The research activities within the project focused on refining computational methods and software developed by researchers at the Institute of Crystallography of CNR for crystal structure determination. Specifically, in the analysis of metal-organic frameworks (MOFs), structural solution procedures were tested on a subset of known structures extracted from the Cambridge Structural Database (CSD). The results demonstrated that real-space methods can be highly effective in solving these structures, provided the data quality is sufficiently high and an accurate structural model of the organic linkers and secondary building units is available. Additionally, the CNR-IC Unit collaborated with the UNICAL Unit to characterize the crystal structure of selected products obtained from the studied reactions.
The scientific aspects of the COXSECAT project were presented by Corrado Cuocci and Angela Altomare on February 12, 2025, as part of the seminar series “PRIN Wednesday at IC” organized by IC-CNR. The presentation was attended by IC researchers.
The COXSECAT project has been cited in:
Poster presentation. Title: ‘The Software EXPO for Structure Determination from Powder Diffraction Data: Advances’; Authors: Angela Altomare, Corrado Cuocci, and Mauro De Feudis*; Mauro De Feudis is currently a research fellow at the Institute of Crystallography -CNR (IC), CNR in Bari, supported by funding from the COXSECAT project; The poster was presented at the Italian Crystal Growth 2025 (ICG2025) Conference, Lecce, Italy, 19th-22nd January 2025. It highlighted advanced features we have developed and implemented in the software EXPO, and case studies to demonstrate the successful application of Direct Methods and Simulated Annealing in characterizing microcrystalline structures of varying types and complexities, particularly in the case of metal-organic frameworks (MOFs).
Article submitted to Acta Crystallographica A, in review. Title: ‘The Phase-Seeding method for solving non-centrosymmetric crystal structures: a challenge for AI’; Authors: Benedett Carrozzini, Liberato De Caro, Cinzia Giannini, Angela Altomare*, Rocco Caliandro; The paper proposes a new phasing method designed for AI integration to solve crystal structures ab initio, regardless of complexity or symmetry.


LISOC (Laboratory of Industrial and Synthetic Organic Chemistry)
